Technology
We are the sole producer of active recombinant selenoproteins
And with our technology we could theoretically produce any of the 800+ natural selenoproteins yet identified in nature, or any synthetic selenocysteine-containing proteins on demand.
Our recombinant production system uses bacteria
Making the production cost lower compared to use of mammalian systems, and allows us to offer selenoproteins at competitive prices compared to selenoproteins purified from native sources.
Our edge-cutting technology results from 30+ years of extensive research at Karolinska Institutet in Stockholm, Sweden, now making selenoproteins available for academic research and the global Pharma and R&D sectors.
The use in our selenoprotein production technology of E. coli strains lacking RF1 and any endogenous use of TAG/UAG codons is enabled under a license agreement with Harvard College.
For further background information
Please don’t hesitate to get in touch with us!
Key References
Overexpression of Recombinant Selenoproteins in E. coli
Cheng Q and Arnér ESJ.
Methods Mol Biol 2018.
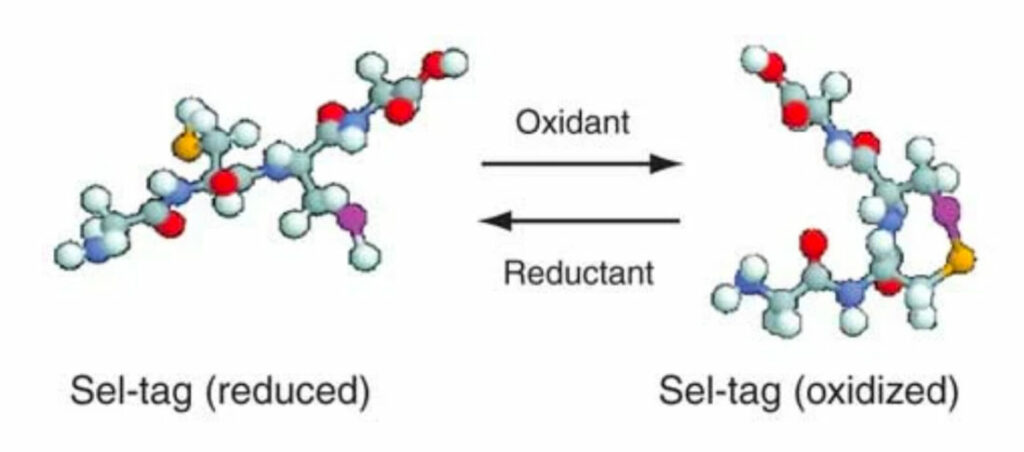
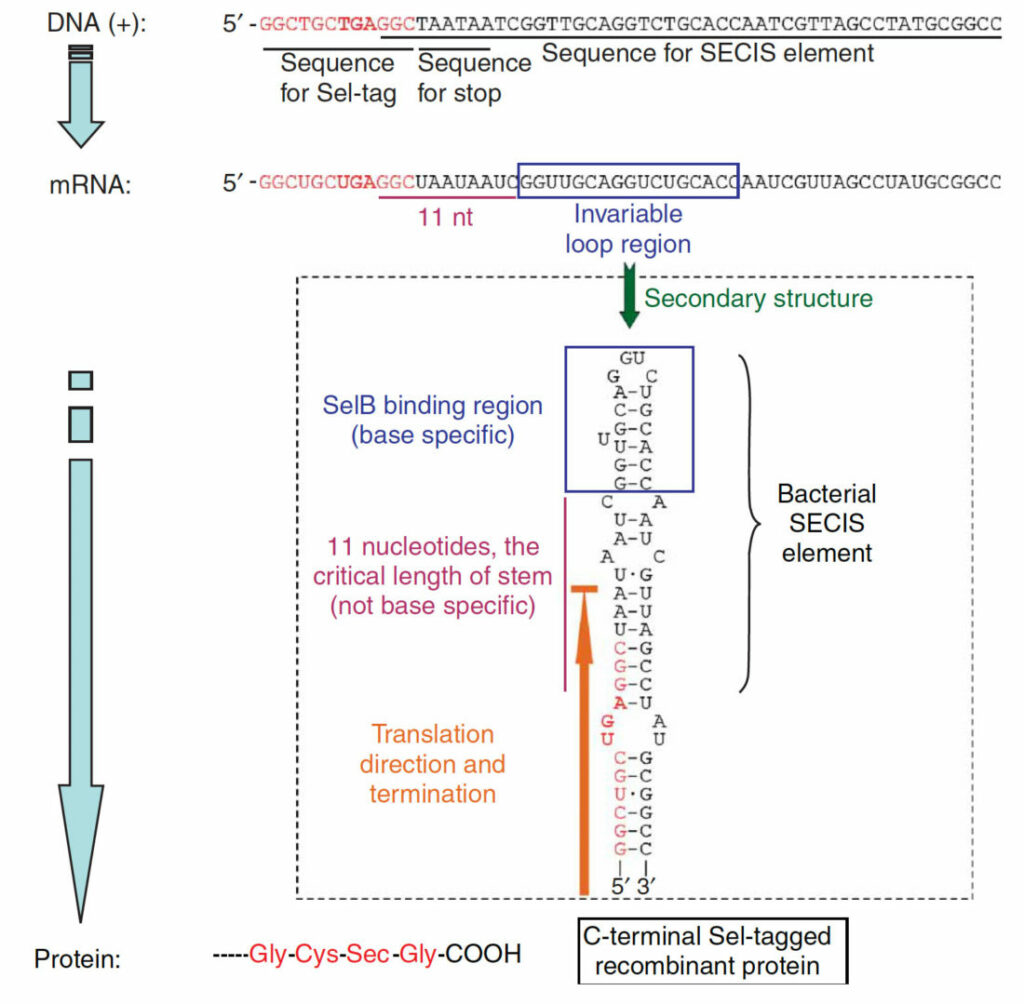
Tagging recombinant proteins with a Sel-tag for purification, labeling with electrophilic compounds or radiolabeling with 11C
Cheng Q, et al.
Nat Protoc 2006.
Exploiting the 21st amino acid – purifying and labeling proteins by selenolate targeting
Johansson L, et al.
Nat Methods 2004.
Latest news
Groundbreaking study challenges present view of ferroptosis mechanisms
Cutting-edge methods for recombinant selenoprotein production have enabled an astonishing discovery by the Arnér group at Karolinska Institutet in Stockholm. By utilizing high quality selenoproteins, with specific activity very close to that of native enzymes, the group successfully established a comprehensive screen for inhibitors of human
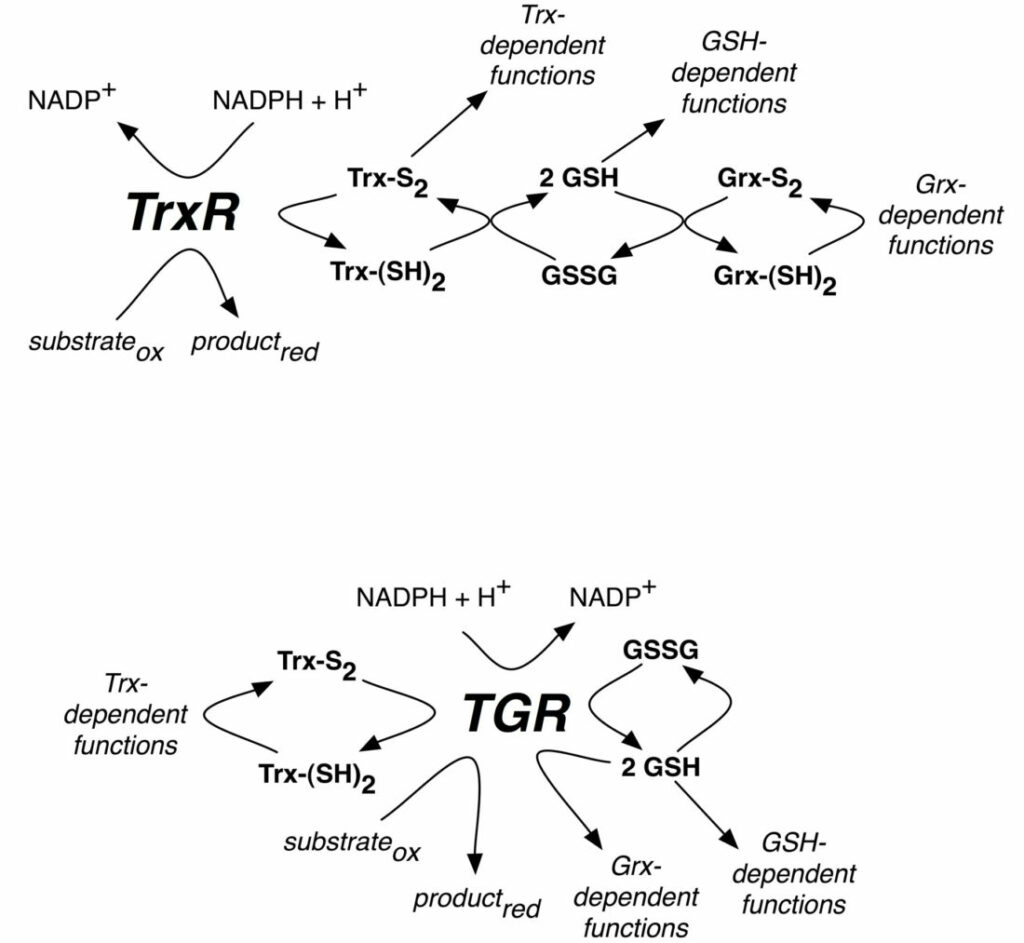
Scheme of reactions catalyzed by mammalian TrxR or parasite TGR selenoproteins
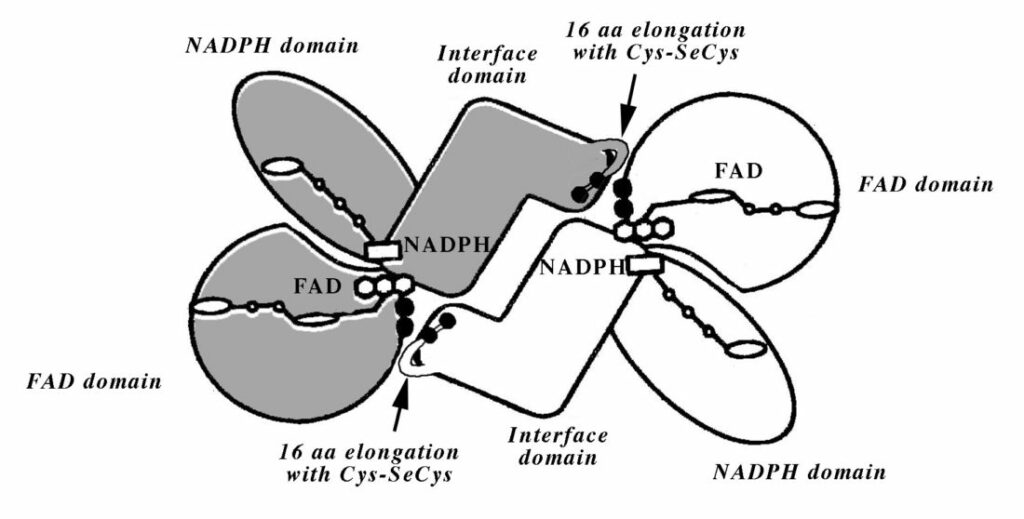
Model of the selenoenzyme TrxR architecture, with its C-terminal arm containing Sec
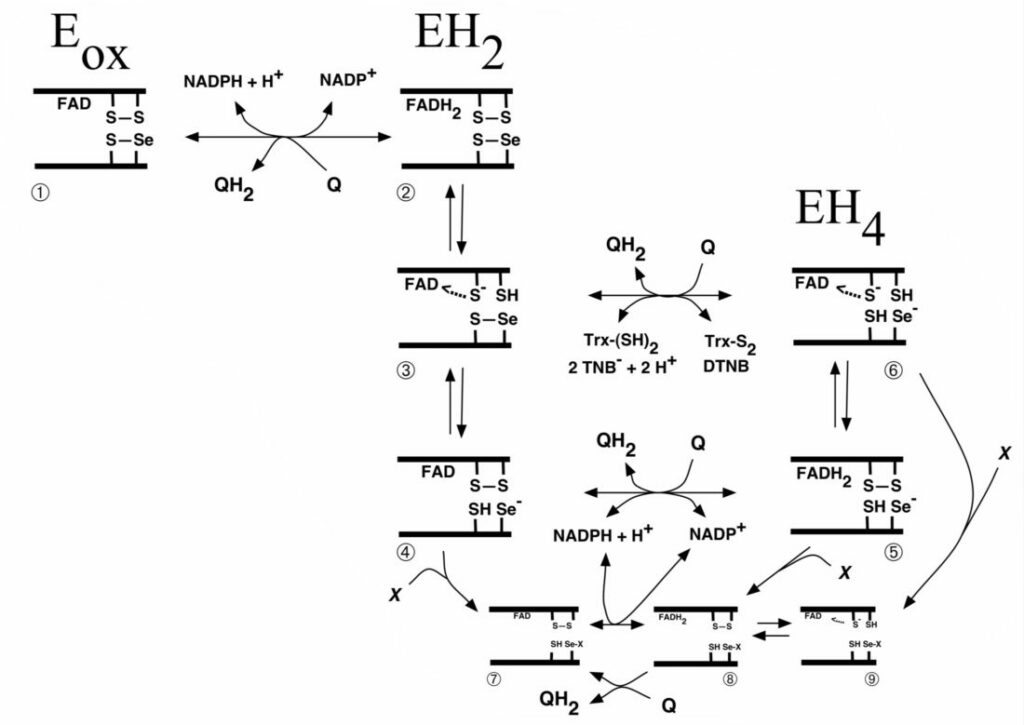
Scheme of quinone interactions with the Sec-containing active site of TrxR
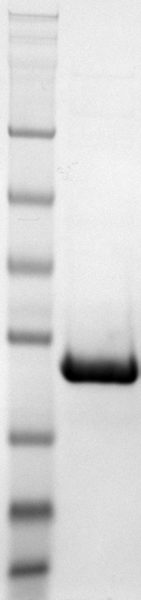
Coomassie-stained SDS-PAGE with pure GPX1 made by Dr. Qing Cheng, CTO at SeLENOZYME
In-house photo
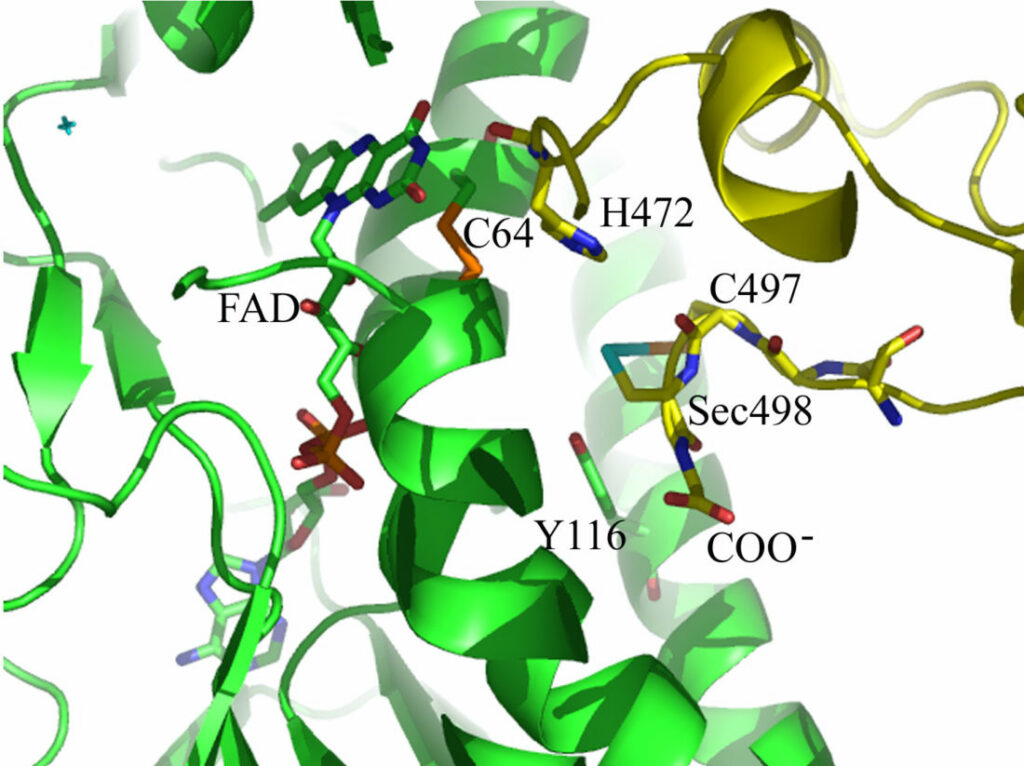
Model of the C-terminal Sec-containing site of TrxR1 as based on the crystal structure